
Package integrity directly affects product sterility, stability and shelf life. Leak testing methods must therefore be reliable, repeatable, and aligned with regulatory expectations.
CDV Pharma V&P supports pharmaceutical laboratories and quality teams in performing consistent package integrity testing across multiple packaging formats and test methods.
A versatile leak detection system for pharmaceutical applications
CDV Pharma V&P is a package integrity testing system designed to perform dye penetration, vacuum, and pressure-based leak tests. Its architecture integrates:
- An optimized vacuum chamber for greater packaging flexibility.
- An independent control module to improves delivery times, simplifies maintenance, and protects sensitive electronics from immersion fluids.
- A PLC-based automation platform.
This design ensures process stability, protection of electronics, and operational flexibility in daily laboratory use.




Multiple leak test methods
One instrument
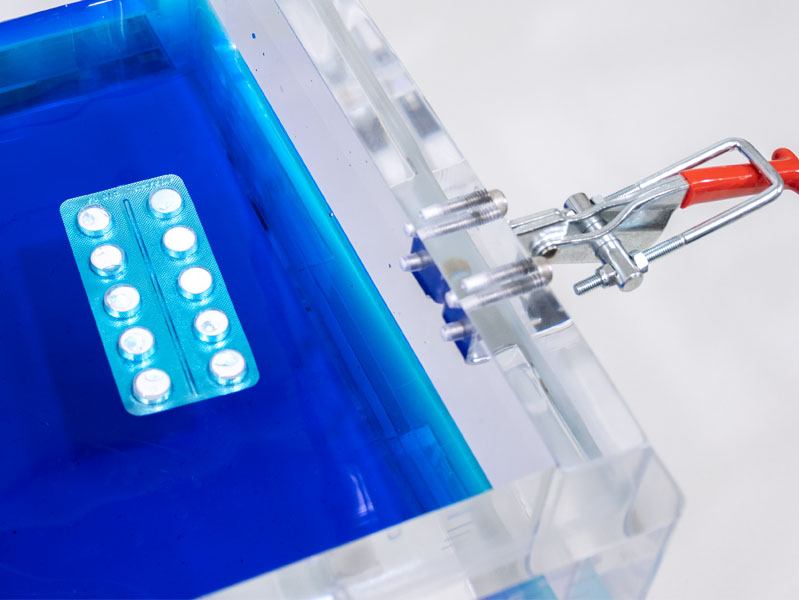
CDV Pharma V&P supports a wide range of package integrity test methods, including:
- Dye penetration testing using methylene blue
- Vacuum-based leak detection
- Internal pressure testing
- External pressure testing for increased sensitivity
With a single system, laboratories can perform different CCIT methods depending on package type and test objectives.

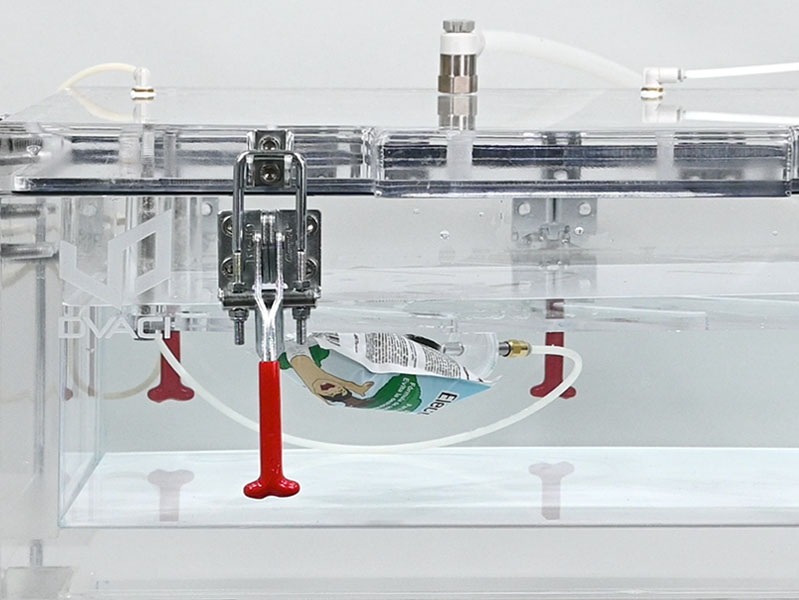

Applications
CDV Pharma V&P is suitable for leak detection testing of:
- Ampoules
- Vials
- Bottles
- Blisters
- Flexible and semi-flexible packages
- Sealed pharmaceutical containers
Tests can be performed on rigid, semi-flexible, and flexible packages, with or without liquid content.
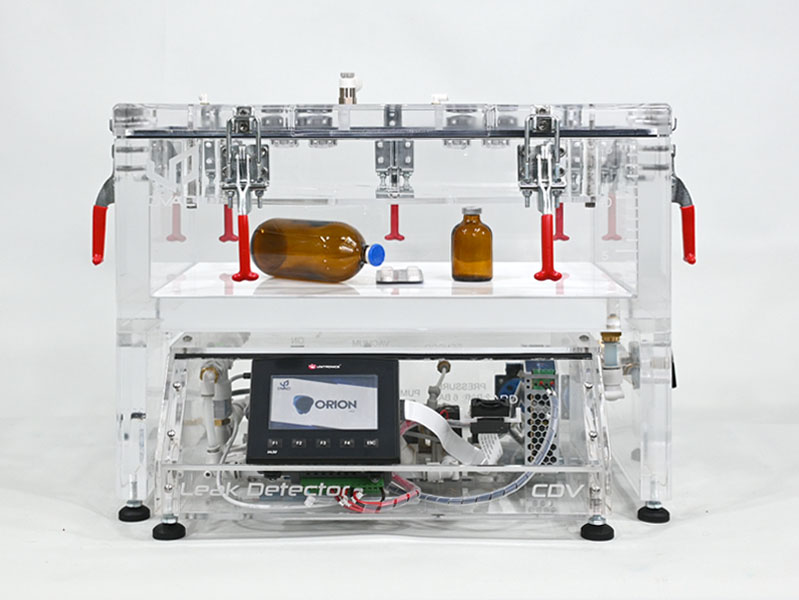
Designed for regulatory compliance

CDV Pharma V&P supports testing aligned with internationally recognized standards, including:
- USP <1207.2>
- USP <381> / JP 7.03
- ASTM D3078
- ASTM F2096
- ASTM D4991
- ASTM D6653
- European Pharmacopoeia 3.2.9
- MGA 0486 Mexican pharmacopoeia (FEUM)
- 21 CFR Part 11 (optional)
This makes CDV Pharma suitable for regulated pharmaceutical quality control environments.
Data integrity and traceability
The PLC-based control system supports:
- User and permission management
- Automatic storage of test results
- Secure PDF report generation (electronic signature optional)
- Digital data export
- Integration with external systems (printers, barcode and QR readers)
These capabilities support data integrity and traceability requirements in pharmaceutical laboratories.

Adapted to real production
and QC environments

CDV Pharma V&P features:
- Increased chamber height for greater packaging flexibility
- Compact internal volume to reduce dye consumption
- Independent control module for improved ergonomics and maintenance
- Clear visual inspection during the entire test cycle
The modular design allows configuration changes and future upgrades.
We are already helping them:








